Testing
Real-World Efficacy Testing Guidelines
Table of Contents (click to open)
1.1 | Physical Testing Chamber Setup for Duct Mounted Devices
1.2 | Mechanical (Fan and Ducting) Setup for Test
1.5 | Temperature and Relative Humidity Setup and Sampling
1.6 | Ion Concentration Sampling
1.8 | Microorganism Composition and Aerosolized Particle Size
1.9 | Particle Count Measurements Size
Downloads
Real-World Efficacy Testing Guidelines
Microorganism Efficacy Testing Guidelines for Duct Mounted Air Cleaning Devices
PuriFi Labs’ Scientific Advisory Board covers the advances in research and discovery that are changing our understanding of air purification devices and shaping real-world efficacy test standards. Science-based facts will help standardize methods for testing used for comparing the real-world efficacy of various technologies such as UV, ionization, air filtration, and ventilation against airborne pathogens and particulates.
1.1 | Physical Testing Chamber Setup for Duct Mounted Devices
1.1.1
Testing should be conducted in a chamber approximately the size of a “normal” room or larger (i.e., small office or single-family residence). Examples of suitable sized chambers are:
a) EPA Aerobiology Chamber, Volume 24.3 m3 (860.0 ft3); (length x width x height, 320.0 x 360.6 x 211.0 cm)1, 2
b) Bespoke constructed testing chambers, with nominal volumes of 28m3 (1000 ft3) to 85m3 (3000 ft3) with nominal ceiling heights of 2.5m (8 ft) to 3m (10 ft).
c) Bespoke constructed home-sized chambers, with nominal volumes of 340m3 (12,000 ft3) with nominal ceiling heights of 2.5m (8 ft) to 3m (10 ft).
1.1.2
Locations of sensors should be consistent with where human occupants would most likely be exposed to microorganisms.
a) Portable sensor stands for the room should be constructed to allow positioning of sensors at key levels, such as the typical height of a person’s head while standing, nominally 1.7m (5.5 ft) or sitting at a desk, nominally 1m (3.5 ft).
b) Simulated furnishings, constructed to be non-particle shedding and non-gassing, may be located in the chamber, where possible, to provide pseudo-realistic obstructions typically found in residential rooms, hospital rooms, and offices.
1.1.3
Auxiliary mixing fans located inside the chamber should be positioned to disperse the air so that it is adequately mixed but not operated in a manner that causes artificial suspension of large particles that would normally be expected to settle or artificial inactivation of smaller particles due to evaporation effects or tension from the air water interface (AWI).
a) Auxiliary mixing fans should be located in the four corners of the chamber, approximately at 0.5m to 1.5m (1.5 to 5 ft) height, oriented horizontally towards the central point.
b) The total output capacity of the collective fans should be approximately 1-3 times the calculated Air Changes per Hour (ACH) being used for each test.
c) To account for real-world behaviors, nominal air velocities for the fans should be in the range of average indoor walking speeds, divided by four, to account for 50% average sitting time and the temporary air movement of humans vs. the constant flow of mixing fans (i.e., 1 m/s, 197 FPM, divided by four, 0.25 m/s or 49.25 FPM) 3

1.2 | Mechanical (Fan and Ducting) Setup for Test
1.2.1
The mechanical aspects of the test chamber should be constructed in a manner that will allow for mounting and use of the air cleaning device consistent with the manufacturer’s recommendations and real-world conditions. Therefore, while efficacy will be measured in the simulated breathing zone, duct-mounted devices (ionizers, UV lights, in-duct air filters) should be duct mounted, and tabletop or portable units should be positioned appropriately. The quantity and manner of operation of the devices should be consistent with the manufacturer’s recommended use and the manner in which they will be reasonably installed and used in a real-world environment.
1.2.2
The supply airflow distribution should be installed in a manner consistent with typical construction methods.
a) Variable flow supply (recirculation) fan, sized to provide 1 to 10 air exchanges per hour, is preferably located exterior to the chamber, if possible. However, biosafety protocols may dictate that all fan systems and ductwork must be fully contained within the chamber.
b) Particulate air filter housing is mounted adjacent to the fan, on the discharge side of the supply fan for commercial applications. For residential applications, a particulate air filter housing might be required at the room side return air register(s).
c) Section of duct suitable to mount an air cleaning device, consistent with the manufacturer’s recommendations, is located approximately 0.5m to 2m (1.5 ft to 6 ft) downstream from the supply fan, depending on device type and space constraints. Sample ports should be located before and after the air cleaning device for additional measurements and analysis as needed.
d) Typically, the main supply air ducting would be approximately 6 to 12m (20 to 40 ft), with multiple 1 to 2m (3 to 6 ft) long branches extending off of the main duct inside the test chamber, terminating in standard 0.1 to 0.3m (6 to 12 in) diffusers, uniformly spaced within the test chamber ceiling, according to typical indoor environments. More diffusers and associated ducting should be installed if the flow through any diffuser exceeds approximately 120 CMH (70 CFM).
e) The ducting is preferably spiral galvanized ducting most often found in residential, commercial, and healthcare installations.
1.2.3
The return airflow should be controlled in a manner consistent with typical installations.
a) Three damper-operated return air grills, one located near the floor, one near the mid-point of the wall, and the other near the ceiling, are connected by ductwork to the externally mounted fan. The air return near the ceiling is preferable in testing. The other air returns can be helpful in evaluating the settling effects of microorganisms.
b) Leading from the return air grills there is a transition piece of ducting leading to the supply fan. Sample ports should be located approximately 0.3m (1 ft) upstream of the supply fan for additional measurements and analysis as needed.

Mounting and use of the air cleaning device should be consistent with the manufacturer’s recommendations and real-world conditions.

The main supply air ducting would be approximately 6 to 12m (20 to 40 ft), with multiple 1 to 2m (3 to 6 ft) long branches extending off of the main duct inside the test chamber.
1.3 | Airflow Balance
1.3.1
The airflow pattern in the test chamber should be adjusted, using the adjustable dampers and the variable flow recirculation fans to ensure, as reasonably as possible, that the treated air coming through the dampers is distributed uniformly throughout the room in coordination with the auxiliary fans.
a) Flow visualization, as used in cleanrooms, is one technique that can be employed.
b) CO2 injection into the recirculation air and periodic measurements at the points throughout the room can be used to assess the uniformity of the air within the room.4
c) An airflow grid can be used to determine flow velocity and volume flow at the dampers, registers, and in the space to ascertain that the air is being uniformly distributed.

The airflow pattern in the test chamber should be adjusted, using the adjustable dampers and the variable flow recirculation fans to ensure, as reasonably as possible, that the treated air coming through the dampers is distributed uniformly throughout the room in coordination with the auxiliary fans.
1.4 | Air Sampling Setup
1.4.1
The air sampling aspects of the testing should take into consideration both the locations within the standing and sitting breathing zones and the number of locations that can reasonably be justified.
a) For efficacy measurements, air sampling at one or more predetermined points will be needed to draw the contaminated particles into an externally located instrument or sampling device.
b) The air drawn from the sampling points within the room during the testing period should not exceed 1% of the total room volume so that the pressure in the room does not cause significant infiltration of outside air.
c) The collection speed of air drawn through the sampling ports should be adjusted for the microorganism involved to ensure collection with the least amount of damage to the microorganism during the collection process. For example, enveloped viruses, such as SARS-CoV-2, may be best collected at approximately 5 LPM, whereas some bacteriophage surrogates might be compatible with faster collection speeds.
1.4.2
The air passing through the recirculation fan should be a well-mixed sample of the air being drawn from the room, and sampling on either the inlet or discharge of the fan, but before the filter or air cleaning device, will give data representative of the overall air in the chamber in addition to specific location data.
1.4.3
The air can be sampled both before and after the duct-mounted filter to determine the single-pass efficiency changes because of the air filter, or because of the air cleaning device, or a combination of both.
1.4.4
The air can be sampled both before and after the air cleaning device to determine the single-pass effects that the air cleaning device is having on specific aspects of the air, such as ion counts, microorganism counts, particle size counts and distribution.
1.4.5
In-room particle counters can also be co-located with microorganism collection ports, ion meters, and other air sampling devices as applicable. Collecting multiple data sets from the same in-room locations helps ensure environmental consistency at the collection point for each respective data set.
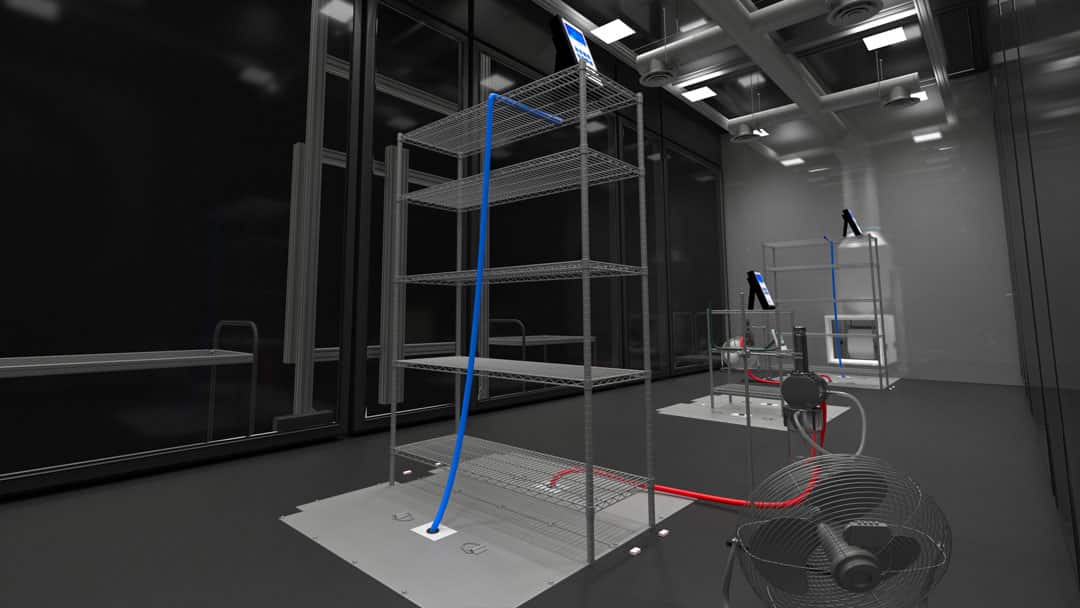
The air sampling aspects of the testing should take into consideration both the locations within the standing and sitting breathing zones.
1.5 | Temperature and Relative Humidity Setup and Sampling
1.5.1
Temperature and Relative Humidity setup and control should be carefully considered as the viability of some microorganisms is affected by relative humidity. Also, consideration should be given to the effect of evaporation rate on the aerosol droplets and that effect on microorganism viability.
a) For example, bacteriophage MS2 is most stable at 30% relative humidity. Testing at 30% relative humidity presents the most challenging conditions for an electronic device to deactivate MS2.5
b) For example, viruses have been reported to survive well at a relative humidity below 33% and at 100%. Low relative humidity could affect the evaporation rate of the aerosol while also affecting particle size and other parameters.6

Bacteriophage MS2 is most stable at 30% relative humidity. Testing at 30% relative humidity presents the most challenging conditions for an electronic device to deactivate MS2.
1.6 | Ion Concentration Sampling
1.6.1
Airborne ion concentration measurements should be logged at a point near air sampling ports being used for specific purposes, such as microorganism analysis.
1.6.2
Ion concentration measurements can be made with several types of instruments.
a) A Gerdian Tube instrument (i.e., “Ion Meter”) measures the air ion density and mobility, sometimes characterized as measuring the air conductivity. The conductivity measurement is not indicative of ions exclusively or the nature of the ions. Any static charge, including ambient charge, charged particles, free electrons, or actual ion species, will contribute to measurements from Gerdian tube-based devices. Because of the requirement that air is drawn through the instrument, it is best used in open spaces.
b) A Langmuir Probe is used for local measurement of plasma parameters because of its small size and simplicity. It is best applied in close proximity to the ionization device discharge.7
c) The most accurate method for measuring and characterizing isolated ion density and excited ion, as well as neutrals, speciation can be accomplished through Optical Emission Spectroscopy as outlined in Item 1.7.1.a.
d) Multiple instruments can be used at different locations to establish distribution of ion concentration and uniformity of ion distribution in the room.

1.7 | Ion Species Sampling
1.7.1
Airborne ion species measurements can be logged at a point near the sample points being used for specific purposes, such as microorganism concentration or device output. The efficacy of the ionization device will likely be related to type (presence of negative and positive ions) and quantity of reactive ion species.
a) Optical Emission Spectroscopy can be used to both identify and quantify many of the species, some by family, other specifically.8, 9
b) Supplemental methods and instruments can be utilized to identify and quantify other species. For instance, the PMS Air Sentry, point of use ion mobility spectrometer (IMS), is reported to have the capability to detect small changes in airborne concentrations (ppt sensitivity) of chlorides, acids, amines, and ammonia-containing species.10
1.8 | Microorganism Composition and Aerosolized Particle Size
1.8.1
The composition of the broth in which the microorganism will be aerosolized can affect the viability of the microorganism. For the specific microorganism used in testing, the appropriate broth should be chosen.
a) Artificial saliva and sputum would appear to be most appropriate.
b) Deionized water can cause issues with the microorganism stability in the aerosolized droplet.11, 5
1.8.2
The particle size at which the aerosolized broth will be generated should be carefully considered and designed to represent the real-world conditions that a given technology is being tested for.
a) Small particles in the 1 to 3 µm range diffuse deep into the lung tissue and deposit by mechanisms including diffusion, sedimentation, and electrostatic forces.
b) Larger particles (>8 µm) impact further up in the respiratory airways.
c) Human aerosols start as low as 0.1 µm but mostly begin around 0.3 µm and hit their peak between 1 and 3 µm, suggesting that exhaled and inhaled aerosols are primarily responsible for human-to-human transmission of microorganisms such as SARS-CoV-2.12, 13, 14, 15, 16
1.8.3
When using a microorganism surrogate, the microorganism surrogate to be selected should closely resemble the target microorganism in both size and structure.
a) Example: Phi6 is a dsRNA phage of the Cystoviridae family that has been suggested as a good surrogate for studying enveloped RNA viruses, including SARS coronaviruses; similar to SARS-CoV-2, it is enveloped by a lipid membrane, has spike proteins, and is of similar size (~ 80 to 100 nm).
b) As a surrogate for non-enveloped pathogens, MS2 bacteriophage might be used.17
Top: MS2 bacteriophage, Bottom left: SARS-CoV-2, Bottom right: Phi6
1.9 | Particle Count Measurements Size
1.9.1
Measurement of the particle counts within the test chamber and mechanical system will be helpful in understanding the effect that the air cleaning device is having on the treated air.
a) Air filter devices will remove certain sized particles from the air, depending on the particle removal efficiency of the filter, based on the ability of microorganisms to reach the air filter device through recirculation.
b) Ionization devices will cause agglomeration of both solid and aerosol (liquid) particles in the air, and the fate of the particles is an important consideration.
c) The decrease of active microorganisms in the air could occur as:
i. the deactivation on a solid particle or inside a liquid particle, or
ii. could be due to the agglomeration and settling of the solid or liquid particles, which may or may not contain viable microorganisms, or
iii. a combination of both deactivation and settling.
1.9.2
Samples will be drawn from the air in the chamber to determine the deactivation of the microorganisms, either from natural decay or induced by device inactivation. The effect of accelerated settling through agglomeration, versus airborne inactivation, is an important factor to consider and account for. To that end, clean deposition coupons can be placed on floors or surfaces to measure the effect of particle agglomeration and settling. The deposition coupons placed inside the chamber can help determine if the reduction of microorganisms in the air is due to airborne inactivation or airborne elimination through the settling of microorganism particles removed from the breathing zone.
1.9.3
The direct particle measurements of microorganism-laden aerosols can be a challenge as they pose a threat to the health and safety of anyone exposed to microorganisms during the test process and servicing of contaminated particle measurement instrumentation. However, as an indirect method of measurement, to develop an understanding of aerosol agglomeration effects and particle physics without active microorganisms, the following optional testing sequenced is proposed:
a) Nebulize the broth (without microorganism) into the test chamber, while operating at the same parameters set for the testing process, but with the air purification device removed or deactivated, and then monitor the particle counts and particle sizes over the estimated period that a test will occur, then;
b) Nebulize the broth (without microorganism) into the test chamber while operating at the same parameters set for the testing process, with the air purification device re-installed or activated, then monitor the particle counts over the same estimated period, as in (a), that the test will occur. The difference in the particle counts and particle sizes will be the settling, agglomeration, or removal effect of the air purification device. An assessment can be made as to the agglomeration effect on the suspension and settling of the aerosol, in essence establishing a material balance for the aerosol.
c) For the ionization deactivation testing, nebulize the broth (with the microorganism) into the test chamber with the ionizer on that is being operated at the prescribed testing parameters. By using the aerosol material balance information established in (b) then adjustments can be applied to the airborne deactivation due to ionization.
1.9.4
Both airborne inactivation and microorganism particle reduction in the breathing zone, including agglomeration effects, should be viewed as positive effects on reducing active microorganism-laden aerosols in the air. Measuring results in this manner ensures that all devices are being measured for their most important impact area, the breathing zone. Studies have documented that ions from cold plasma devices will deactivate microorganisms within the liquid aerosols that have settled onto various hard surfaces.18
1.10 | Viable Microorganism Measurements
1.10.1
Continuous measurement of the viable microorganism counts within the test chamber and mechanical system can be accomplished using specialized instrumentation.
a) The Air Trace® Environmental Air Sampler, made by PMS, is a slit-to-agar microbial air sampler.
b) The Air Trace rotates a 150.0mm agar plate through 360° while the agar plate is maintained at a fixed distance from a precision slit, ensuring maximum validated recovery of impacted organisms on the agar surface.
c) After the inoculated plate has been exposed, growth on the medium can be interpreted, correlating when the contamination occurred during the sampling cycle, enabling pinpointing a particular event, such as 99% reduction point, during a testing period.
d) The user can set the rotation speed of the agar plate, and the percentage of the plate area exposed, thus ensuring the flexibility to monitor the level of biological activity during the testing process.
e) The airflow through the Air Trace instrument is automatically controlled at 1.0 CFM (28.3 LPM).19
1.10.2
Semi-continuous measurement of the viable bacteria or virus counts within the test chamber and mechanical system can be accomplished using biosamplers.
a) Biosamplers are connected to air sampling pumps that draw air at a rate of 12.5L of air sampled during each sampling period.
b) Bioaerosol samples should be taken in at least two representative locations, from the approximately 1.7m (5.5 ft) “breathing zone” height during each sample period.20
c) The broth solution from the impinger is sampled and analyzed for microorganism content.
1.10.3
Total time and discrete-time sampling to measure the microorganism levels can be taken inside the test chamber using deposition coupons that can be uncovered and recovered after a predetermined time.
a) Cleaned stainless steel coupons are typically used.
1.10.4
Deactivation coupons can be used to assess the deposition of viable microorganisms occurring during the test.
a) Some settling of large aerosol droplets is expected during the nebulization of the microorganism broth.
b) Deposition of viable microorganisms due to agglomeration is also expected.
c) Simultaneously deactivation of settled microorganisms on the chamber surfaces due to the presence of the ions is also expected.
d) Testing with and without the ionizer on will help in quantitatively determining the deactivation effect.
1.10.5
Collection methods and speeds should be carefully aligned to the microorganism being tested.
a) Example 1: Enveloped viruses, such as SARS-CoV-2, are susceptible to sampling damage if collected too rapidly (i.e., >5 LPM).
b) Example 2: Bacteriophage surrogates, non-enveloped surrogates in particular, such as MS2, are generally able to be sampled at higher speeds than non-enveloped viruses (i.e., ≥12.5 LPM).
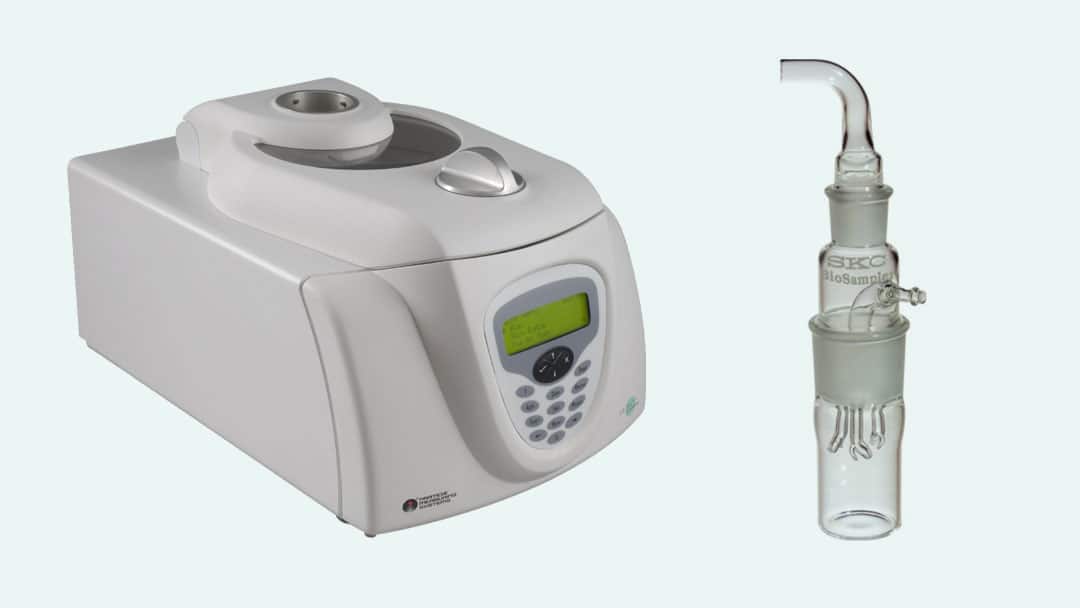
Left: The Air Trace® Environmental Air Sampler continuously measures the viable microorganism counts within the test chamber and mechanical system. Right: The BioSampler® collects bioaerosols in liquid for sample times up to eight hours when used in connection with the sonic-flow BioLite+ pump.
Citations
1: S. Sattar, et al. Decontamination of indoor air to reduce the risk of airborne infections: Studies on survival and inactivation of airborne pathogens using an aerobiology chamber. American Journal of Infection Control, 2016 Oct 1;44(10):e177-e182. doi: 10.1016/j.ajic.2016.03.067. Epub 2016 Jun 30.
https://pubmed.ncbi.nlm.nih.gov/27375064/
2: B. Zagar, et alMathematical modeling and simulation of bacterial distribution in an aerobiology chamber using computational fluid dynamics. American Journal of Infection Control, 44 (2016) S127-S137 https://pubmed.ncbi.nlm.nih.gov/27590697/
3: Jacobs PG, Wan EA, Schafermeyer E, et al. Measuring in-home walking speed using wall-mounted RF transceiver arrays. Annu Int Conf IEEE Eng Med Biol Soc. vol. 2014 (2014):914-917. doi:10.1109/EMBC.2014.6943740 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4288466/
4: Yicheng Zeng, Prashik Manwatkar, Aurélie Laguerre, Marina Beke, Insung Kang, Akram S. Ali, Delphine K. Farmer, Elliott T. Gall, Mohammad Heidarinejad, Brent Stephens. Evaluating a commercially available in-duct bipolar ionization device for pollutant removal and potential byproduct formation, Building and Environment, Volume 195 (2021) 107750, ISSN 0360-1323.doi.org/10.1016/j.buildenv.2021.107750. https://www.sciencedirect.com/science/article/pii/S036013232100158X
5: Z. Zuo, et al. Survival of Airborne MS2 Bacteriophage Generated from Human Saliva, Artificial Saliva, and Cell Culture Medium. Appl Environ Microbiol. 2014 May; 80(9): 2796-2803. doi: 10.1128/AEM.00056-14 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3993287/pdf/zam2796.pdf
6: Lin K, Marr LC. Humidity-Dependent Decay of Viruses, but Not Bacteria, in Aerosols and Droplets Follows Disinfection Kinetics. Environ Sci Technol. 2020 Jan 21;54(2):1024-1032. doi: 10.1021/acs.est.9b04959. Epub 2020 Jan 10. PMID: 31886650. https://pubmed.ncbi.nlm.nih.gov/31886650/
7: C. Lacdan, M. Wada. Characterization of Atmospheric Pressure Plasmas by a Gerdien Condenser. Plasma and Fusion Research: Regular Articles, Volume 11, 2401015 (2016): 2401015-1 – 2401015-4. doi: 10.1585/pfr.11.2401015 http://www.jspf.or.jp/PFR/PDF2016/pfr2016_11-2401015
8: X.Cheng et al. The Effect of Tuning Cold Plasma Composition on Glioblastoma Cell Viability, PLoS ONE. Vol. 9 (2014) Issue 5: e98652. doi.org/10.1371/journal.pone.0098652. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0098652
9: Li Lin, Yuanwei Lyu, Barry Trink, Jerome Canady, and Michael Keidar , “Cold atmospheric helium plasma jet in humid air environment”, Journal of Applied Physics 125, 153301 (2019) https://doi.org/10.1063/1.5086177
11: Maohua Pan, Leah Carol, John A. Lednicky, Arantzazu Eiguren-Fernandez, Susanne Hering, Z. Hugh Fan & Chang-Yu Wu (2019) Determination of the distribution of infectious viruses in aerosol particles using water-based condensational growth technology and a bacteriophage MS2 model, Aerosol Science and Technology, 53:5, 583-593, https://www.tandfonline.com/doi/full/10.1080/02786826.2019.1581917
12: M Z. Bazant and J. W. M. Bush. A guideline to limit indoor airborne transmission of COVID-19, PNAS (2021) Vol. 118 No. 17 e2018995118. https://doi.org/10.1073/pnas.2018995118. https://www.pnas.org/content/pnas/118/17/e2018995118.full.pdf
13: R. J. Thomas. Particle size and pathogenicity in the respiratory tract, Virulence. (2013) 4:8, 847-858, https://doi.org/10.4161/viru.27172.
14: G.R.Johnson, L.Morawska, Z.D.Ristovski, M.Hargreaves, K.Mengersen, C.Y.H.Chao, M.P.Wan, Y.Li, X.Xie, D.Katoshevski, S.Corbett. Modality of human expired aerosol size distributions, Journal of Aerosol Science, Volume 42 (2011) Issue 12: Pages 839-851, ISSN 0021-8502, https://doi.org/10.1016/j.jaerosci.2011.07.009. https://www.sciencedirect.com/science/article/pii/S0969212603002223
15: W. L. Dietrich, J. S. Bennett, et al. Laboratory Modeling of SARS-CoV-2 Exposure Reduction Through Physically Distanced Seating in Aircraft Cabins Using Bacteriophage Aerosol, Morbidity and Mortality Weekly Report. Volume 70 (2020) No. 16, 595-599. https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7016e1-H.pdf
16: K. P. Fennelly. Particle sizes of infectious aerosols: implications for infection control. The Lancet Respiratory Medicine. Volume 8 (2020) Issue 9: P914-924.
https://doi.org/10.1016/S2213-2600(20)30323-4.
https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30323-4/fulltext
17: A. Fedorenko, M. Grinberg, T. Orevi, et al. Survival of the enveloped bacteriophage Phi6 (a surrogate for SARS-CoV-2) in evaporated saliva microdroplets
deposited on glass surfaces. Sci Rep 10, 22419 (2020). https://doi.org/10.1038/s41598-020-79625-z.
https://www.nature.com/articles/s41598-020-79625-z
18: V. Stadnytskyi, C. E. Bax, A. Bax, P. Anfinrud. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission, Proceedings of the National Academy of Sciences (2020) 117 (22) 11875-11877; https://doi.org/10.1073/pnas.2006874117.
19: https://www.pmeasuring.com/wp-content/uploads/2019/04/air_trace_environmental_air_sampler_052214.pdf
20: https://www.skcinc.com/products/biosampler-20-ml-3-pieces

